An open-source software analysis package for Microspheres with Ratiometric Barcode Lanthanide Encoding (MRBLEs)
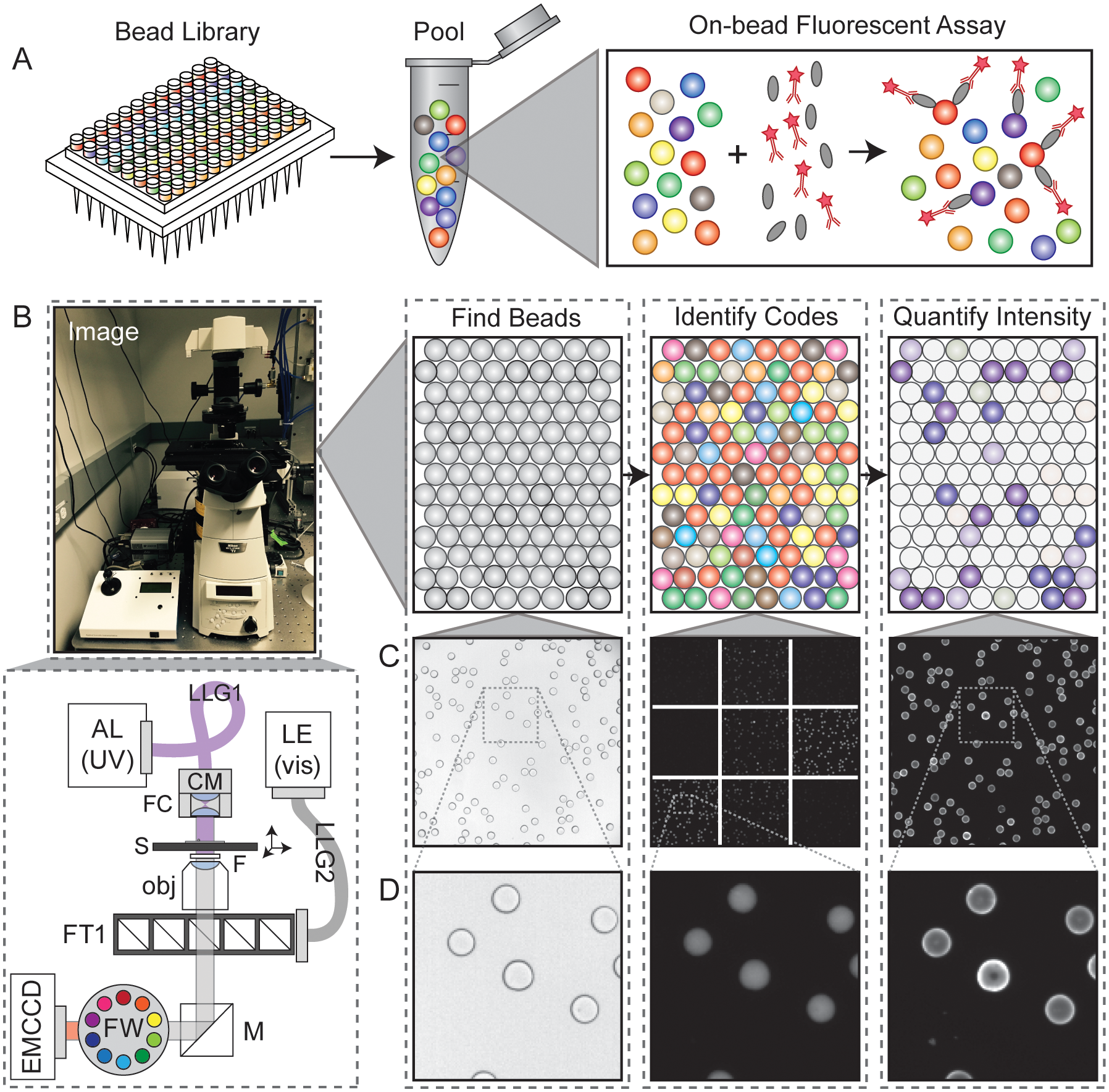
Multiplexed bioassays, in which multiple analytes of interest are probed in parallel within a single small volume, have greatly accelerated the pace of biological discovery. Bead-based multiplexed bioassays have many technical advantages, including near solution-phase kinetics, small sample volume requirements, many within-assay replicates to reduce measurement error, and, for some bead materials, the ability to synthesize analytes directly on beads via solid-phase synthesis. To allow bead-based multiplexing, analytes can be synthesized on spectrally encoded beads with a 1:1 linkage between analyte identity and embedded codes. Bead-bound analyte libraries can then be pooled and incubated with a fluorescently-labeled macromolecule of interest, allowing downstream quantification of interactions between the macromolecule and all analytes simultaneously via imaging alone. Extracting quantitative binding data from these images poses several computational image processing challenges, requiring the ability to identify all beads in each image, quantify bound fluorescent material associated with each bead, and determine their embedded spectral code to reveal analyte identities. Here, we present a novel open-source Python software package (the mrbles analysis package) that provides the necessary tools to: (1) find encoded beads in a bright-field microscopy image; (2) quantify bound fluorescent material associated with bead perimeters; (3) identify embedded ratiometric spectral codes within beads; and (4) return data aggregated by embedded code and for each individual bead. We demonstrate the utility of this package by applying it towards analyzing data generated via multiplexed measurement of calcineurin protein binding to MRBLEs (Microspheres with Ratiometric Barcode Lanthanide Encoding) containing known and mutant binding peptide motifs. We anticipate that this flexible package should be applicable to a wide variety of assays, including simple bead or droplet finding analysis, quantification of binding to non-encoded beads, and analysis of multiplexed assays that use ratiometric, spectrally encoded beads.